Invigorating, bright and vital to life oxygen truly is a wonder of nature. The unassuming element, portrayed with”O” on the periodic table “O” on the periodic table, is not just important for breathing, as well as essential in the design of many chemical molecules. In order to understand the fundamentals of oxygen, it is necessary to be able to examine the atomic structure of it, starting with its electrons, the fascinating small particles that are at in the center of an atom.
The Atomic Composition of Oxygen
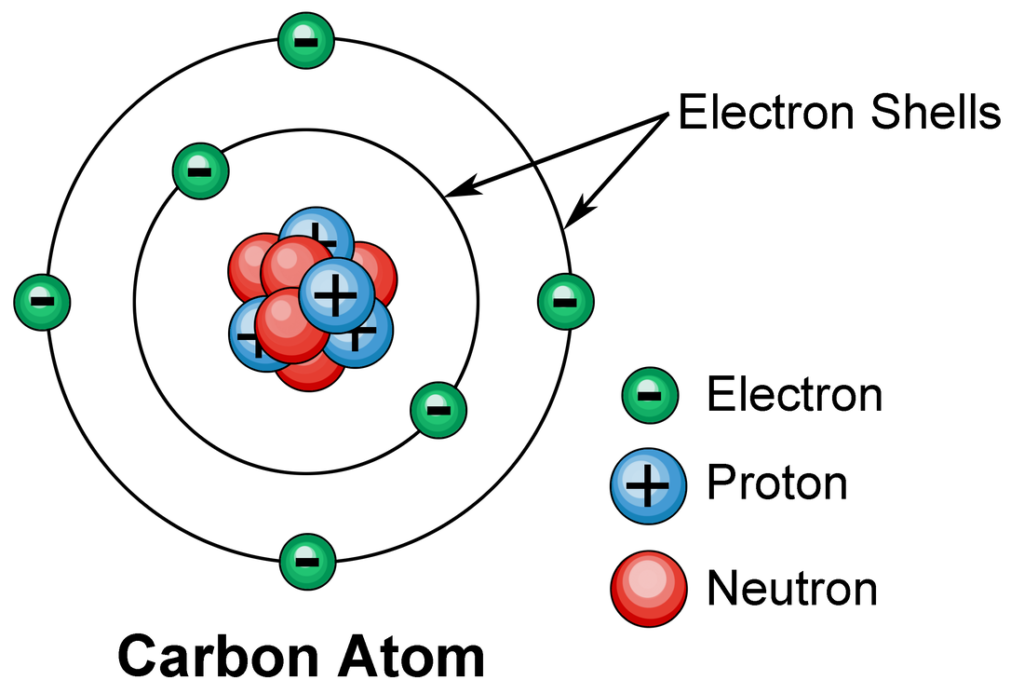
The oxygen atom is located in the 16th category in the periodic table oxygen is the atom with the number 8 in the periodic table. That means an oxygen atom has eight positively charged proton particles within the nucleus. They are balanced with 8 electrons that are negatively charged rotating in different circular orbits about the nucleus. The electrons in nature are the designers drawing the abilities and features of every atom they are a part of.
The electrons inside the oxygen atom are organized according to energy levels or what they call “electron shells.” The first oxygen shell, which is the one located closest to the nucleus can be able to hold up to 2 electrons. Think of it as an intimate dining room table for two. The other 6 electrons have their place within the second, that can accommodate up to eight guests. However, the shell is not completely filled with oxygen’s casing. This particular arrangement with two electrons in the initial shell, and six in the second one gives oxygen ability to be a chemically versatile substance.
Why Electrons Matter
Electrons tell the tale of the journey of an atom. They decide how an individual atom interacts with the surrounding and form bonds, as well as creating molecules, and eventually changing into the wonders observed in nature and industries. The six electrons within its outer shell create a strong desire to be with others. The desire, also known as “chemical bonding tendency,” is a result of the atom’s need to maintain stability by filling its shell with 8 electrons. This is called”the “octet rule.”
The need for oxygen makes it an active player in chemical reactions particularly when it comes to forming compound with other elements. It readily accepts and shares electrons and forms bonds that are ranging between those found in water (H2O) that we drink, as well as the carbohydrate that power the body.
The Role of Electrons in Oxygen’s Behavior
Its electronic structure also provides the behavior of diatomic molecules (O2)–its most stable and widespread shape in the the Earth’s atmospheric. In sharing electrons with another oxygen atom both get the coveted octet structure creating a strong bond enough to keep them to each other. The diatomic oxygen provides the majority of the life on Earth in our lungs, and fueling the internal combustion of cells.
In addition, the electrons in oxygen create an ideal antioxidant. It can pull electrons away from other substances. This is an essential characteristic in metabolic processes, combustion, and even in corrosion.
Fun Fact About Oxygen’s Electrons
Do you realize that oxygen is one of the most stunning trios with its variations in isotopes? O-16, O-17, and O-18 are slightly different in neutron counts, yet all share the same electrochemical ballet of 8 electrons. The results show that although the neutrons and protons of an atom might change, electrons are the core of its character and identity.
Conclusion
The slender elegance of eight electrons reveals the brilliance of oxygen by describing oxygen as life-giving as well as a catalyst for chemical reactions. From the sound of leaves sucking in oxygen to the thunder of the flames that are fueled by it, these tiny electrons perform amazing acts that form the core of our existence. If you are awestruck by the creation of delicate snowflakes or how strong a stainless steel bridge, the electrons of oxygen provide proof of their delicate beauty in the microcosm of.
The best way to comprehend the marvels that surround us, we must understand the art of atoms such as oxygen. Although the electrons of oxygen are tiny yet their contribution to the larger story of chemical and life is truly amazing.
Visit Here Also:- techaitime